Mercury and bromine are the only two elements on the periodic table that are liquid at room temperature.
What two elements on the periodic table are liquid at room temperature.
C identify an element that would tend to gain two electrons.
These elements are liquids because the intermolecular forces are strong enough so that it does not vaporize.
Radon helium xenon neon krypton and argon are eight noble gases.
The melting and boiling points of the halogens increase as you increase atomic number as you move down the periodic table.
Fluorine and chlorine are gases.
Point at or click an element in the periodic table for more information.
Mercury hg and bromine br are the only elements in the periodic table that are liquids at room temperature.
Although elements caesium cs rubidium rb francium fr and gallium ga become liquid at or just above room temperature.
Mercury with a melting point of minus 38 83 c 234 32 k minus 7 89 f and a boiling point of 356 73c 629 88 k 674 11 f is the most well known of these.
Iodine is a solid.
Liquids stp and liquids around room temperature the only liquid elements at standard temperature and pressure are bromine br and mercury hg.
They are nonreactive mono atomic elements with extremely low boiling points.
D identify the group having metal non metal liquid as well as gas at the room temperature.
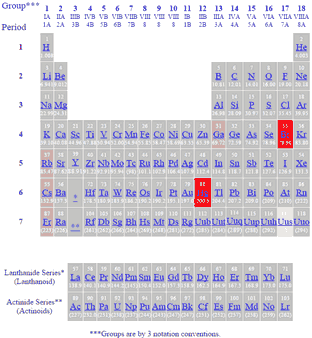
In the periodic table above black squares indicate elements which are solids at room temperature about 22ºc those in blue squares are liquids at room temperature and those in red squares are gases at room temperature.
B identify an element that would tend to lose two electrons.
Most of the metals are solids under ordinary conditions i e 25ºc 1 atmosphere of pressure etc with the exception of mercury hg element 80 which solidifies.
By this definition bromine and mercury are the only two elements that are liquid at room temperature.
For science it s usually considered to be either 20 c or 25 c.
Use the periodic table to answer the following questions.
Three other elements cesium gallium and rubidium become liquid near this mark.
Each of the 13 elements has their own unique physical and chemical properties.
At this temperature and ordinary pressure only two elements are liquids.
A identify an element with five electrons in the outer subshell.
The elements change their state of matter at room temperature and pressure as you increase atomic number.
Elements that are liquid at 25 c.
These two are mercury and bromine.