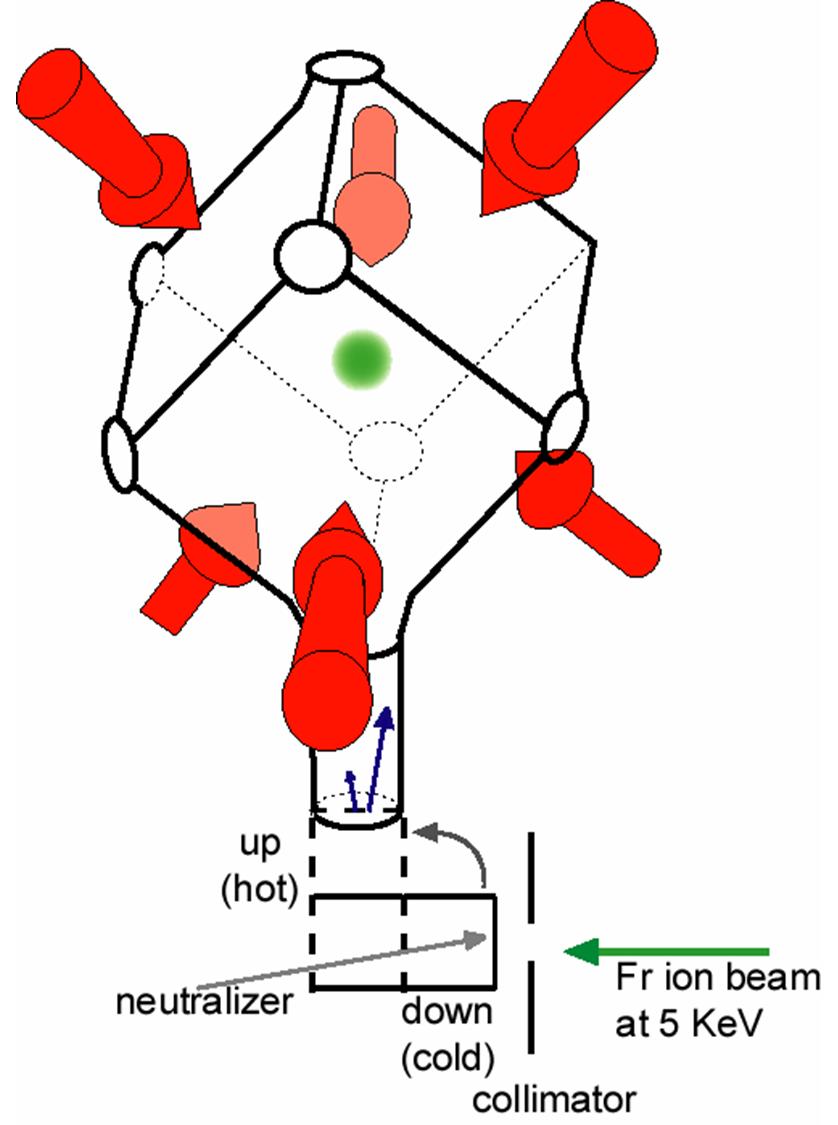
Its most stable isotope francium 223 originally called actinium k after the natural decay chain it appears in has a half life of only 22 minutes.
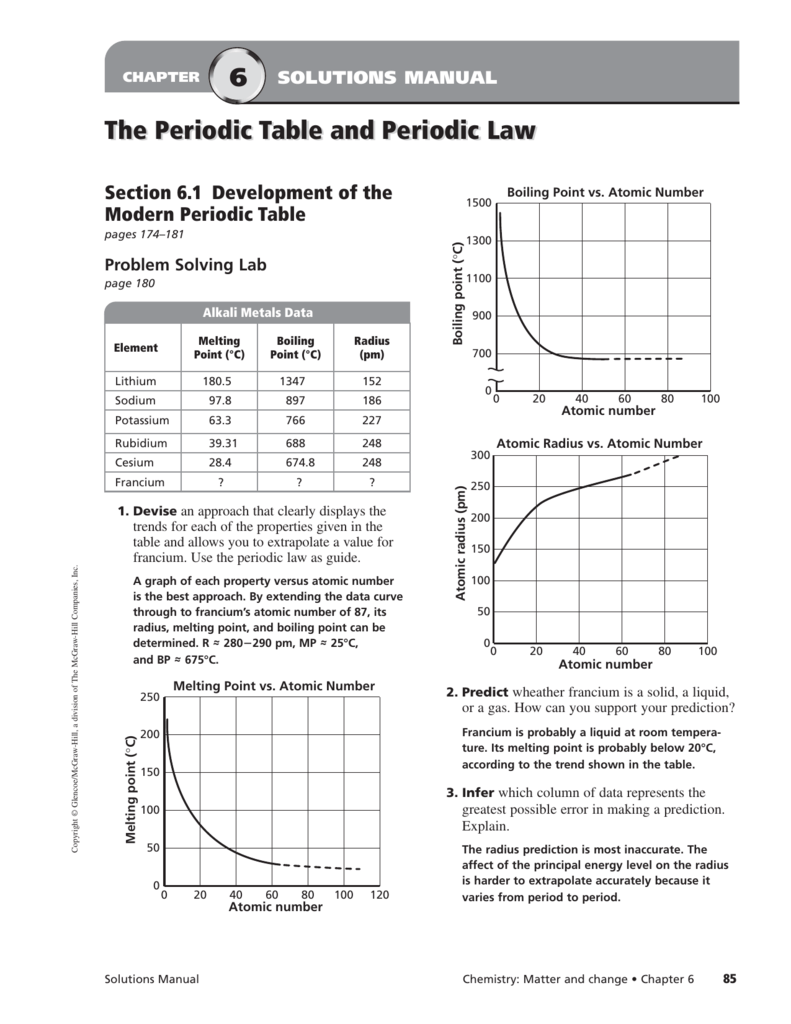
What state would francium be at room temperature.
The temperature at which the liquid gas phase change occurs.
Francium s most stable isotope francium 223 has a half life of about 22 minutes.
Francium is a solid at room temperature.
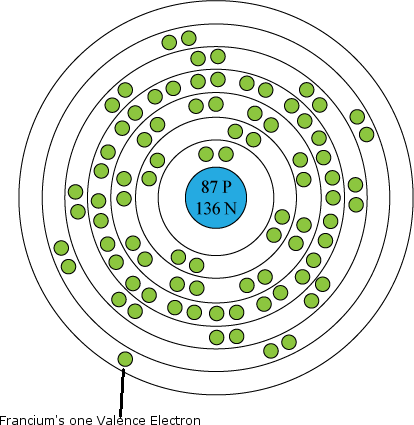
Francium is a chemical element with the symbol fr and atomic number 87.
It s expected the element would be a shiny metal in its pure state like the other alkali metals and that it would readily oxidize in air and react very vigorously with water.
Density g cm 3 density is the mass of a substance that would fill 1 cm 3 at room temperature.
Francium is most likely a solid at room temperature but this cannot be known for certain as it is impossible to gather enough francium in one place to test its physical and chemical properties.
Prior to its discovery it was referred to as eka caesium it is extremely radioactive.
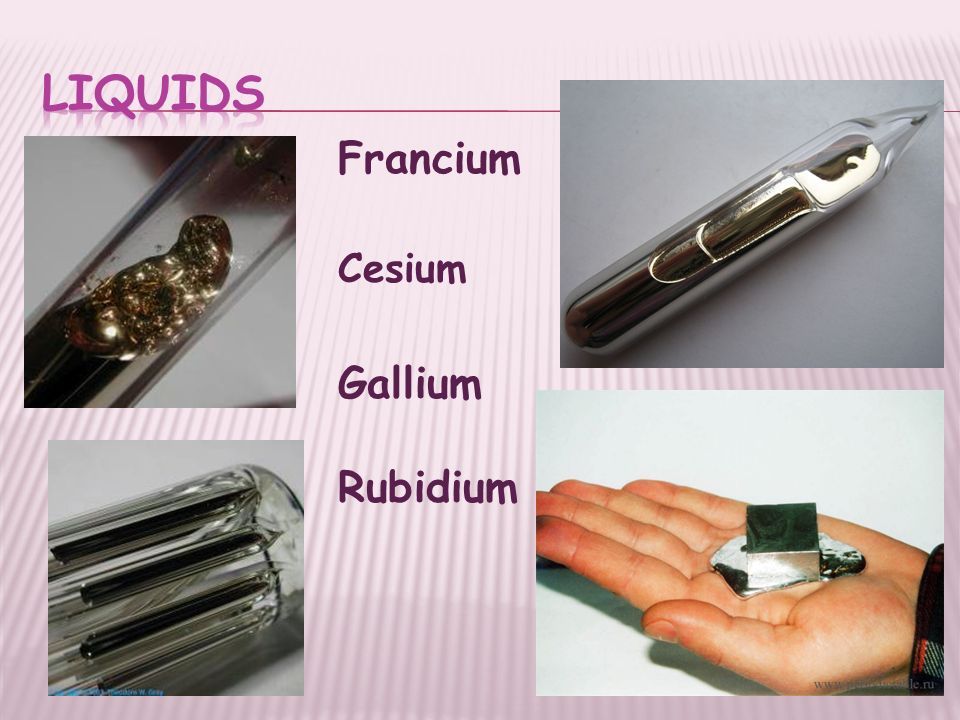
Francium is the shiny metal in its pure state and exist in liquid form at room temperature rather than a solid.
Due to the small amounts produced and its short half life there are currently no uses for francium outside of basic scientific research.
Sublimation the transition of a substance directly from the solid to the gas phase without passing through a liquid phase.
It is considered to be a solid at room temperature 20oc although francium has a very short half life 22 miuntes so the heat and energy given off by its decay may mean it is technically a.
Francium is most likely a solid at room temperature but this cannot be known for certain as it is impossible to gather enough francium in one place to test its physical and chemical properties.
Relative atomic mass the mass of an atom relative to that of.
It is possible that francium may be a liquid rather than a solid at room temperature and pressure.
Find color of francium fr at room temperature or find color of different elements like aluminum barium boron brass bromine bronze cadmium calcium carbon.
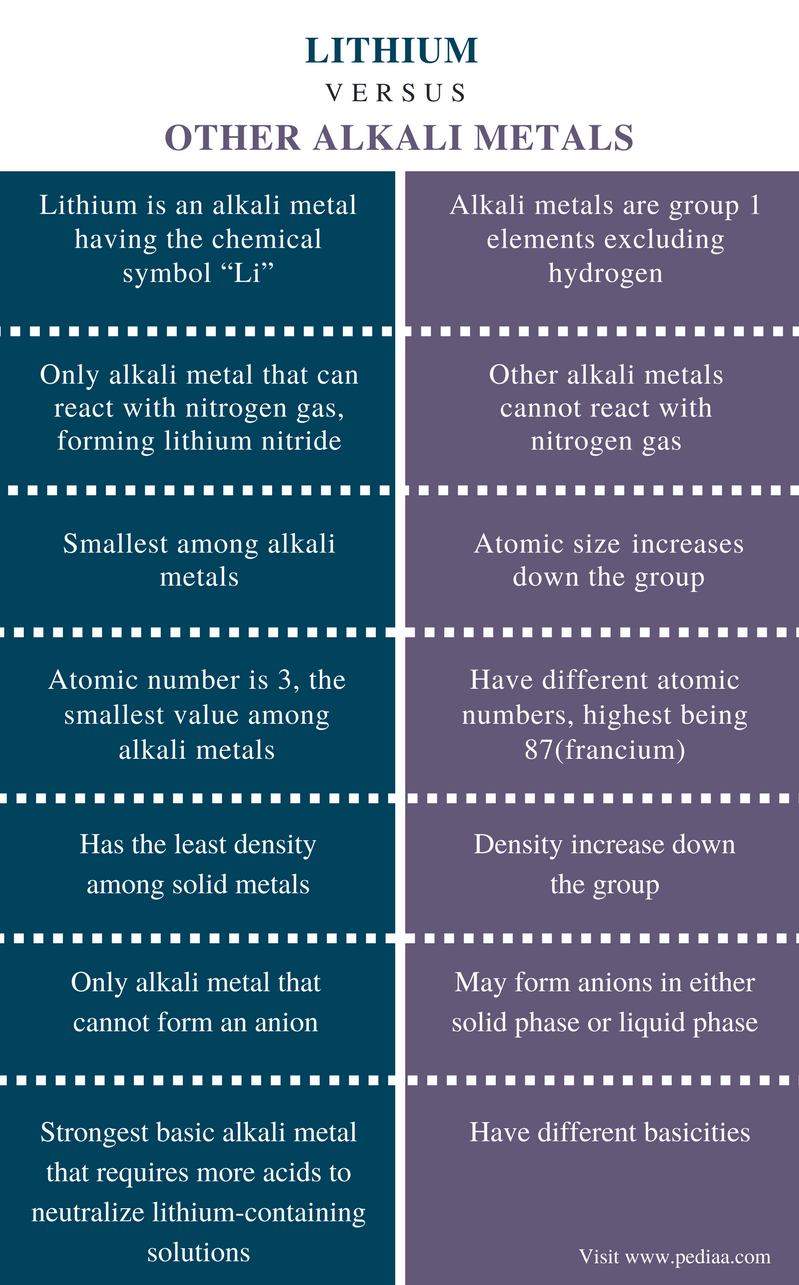
It is the second most electropositive element behind only caesium and is the second.